Applications and Principles of Circular Dichroism Spectroscopy in Protein Secondary Structure Analysis
The secondary structure of proteins refers to the local folding patterns of the protein chain in space, mainly including types such as α-helix, β-pleated sheet, turns, and random coils. These structures are the foundation of the three-dimensional structure of proteins and are crucial for their function.
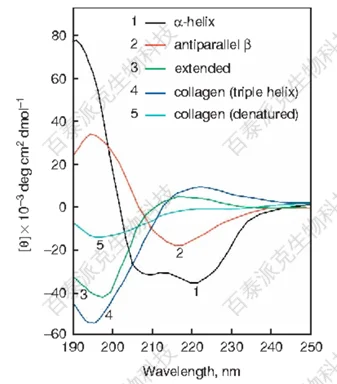 Figure 1
Figure 1
CD spectroscopy provides information about the secondary structure of proteins, as explained below:
1. Differential absorption of polarized light:
CD is a technique that measures the differential absorption of left and right circularly polarized light by molecules. Optically active centers in molecules, such as chiral amino acid residues, cause different absorption of left and right polarized light.
2. Optical activity:
Amino acid residues in proteins are chiral, and their specific spatial arrangement leads to optical activity in proteins, allowing them to interact with circularly polarized light.
3. Specific absorption characteristics of secondary structures:
Different secondary structures of proteins (such as α-helix, β-sheet, β-turn, and random coil) have distinct CD spectral characteristics. These characteristics reflect the specific spatial arrangement of amino acid residues.
Applications
1. Quantitative analysis of secondary structure:
CD spectroscopy can be used for quantitative analysis of the proportions of structures such as α-helix and β-sheet in a protein sample. The absorption intensity at specific wavelengths reflects the relative content of these structures.
2. Monitoring conformational changes:
CD spectroscopy can be used to monitor the effects of changes in temperature, pH, chemical modifications, or interactions (such as ligand binding) on the secondary structure of proteins.
3. Studies on protein folding and stability:
Using CD spectroscopy, researchers can assess the folding state and stability of proteins and observe structural changes during folding and unfolding processes.
4. Structural characterization of new proteins:
For newly expressed or purified proteins, CD spectroscopy provides a quick method to assess their secondary structure.
In summary, circular dichroism spectroscopy is very valuable for understanding how proteins fold and function. However, it usually cannot provide detailed information about higher-level structures of proteins, which may require other techniques such as X-ray crystallography, nuclear magnetic resonance (NMR), or cryo-electron microscopy.
Biotech Pte Ltd - A premium provider of bioproduct characterization and multi-omics mass spectrometry analysis services
Related services:
Circular dichroism (CD) analysis
How to order?






